Socially Distanced Electrons
by Alan S. Brown
Recent discoveries at Kavli Nanoscience Institutes covers everything from quantum mechanics to DNA
The Author
The Researchers
It’s an interesting time for advances in quantum nanoscience, with all the strangeness that implies. We’ve recently learned, for example, how to control the spin of electrons with an acoustic resonator, a microscale device that that can organize electrons by vibrating billions of times per second. Another research team is pursuing another type of mechanical resonator to store and transmit quantum information. Meanwhile, a third group of Kavli scientists are probing the unusual behavior of electrons in materials only a few atoms thick. We also note the potential for making polymers from DNA and some notable honors for two Kavli researchers.
New spin on sensors
How do you build a tiny, super-sensitive sensors? One way is to control the spin of electrons, which are highly sensitive to changes in magnetic fields. Ordinarily, that is done with magnetic fields, but this is bulky. Seven years ago, Greg Fuchs, a member of the Kavli Institute at Cornell, developed a microscale device that vibrated two the three billion times per second to align electrons, but he still needed an external magnetic field to manage some transitions. Now he has found a way to do this solely with the vibrations generated by a microscale resonator. This enables him to control local regions of spin independently within a single chip, something impossible to do with larger magnetic fields. Cornell has patented the technology, which will make possible closely spaced independent sensors within a single chip.
Putting the “mechanics” back in quantum mechanics
When scientists talk about quantum mechanics, they usually mean the interaction of subatomic particles via packets of energy, particle-wave duality, uncertainty, and the like. But Simon Groeblacher, a member of the Kavli Institute of Nanoscience at Delft Technical University, is also talking about achieving quantum interactions using mechanical resonators. He has shown this with a quantum repeater, a device that stores and resends quantum information so that it can travel over long distances. The device he created is the first to show that mechanics can be used as quantum memory in a repeater. Unlike other quantum repeaters, which rely on naturally occurring atomic or rare earth metal resonance, his engineered system enables researchers to work at any wavelength—including low-loss wavelengths used by telecommunications systems to send signals long distances. This is another step forward in the development of “unhackable” secure quantum networks.
Socially distanced electrons
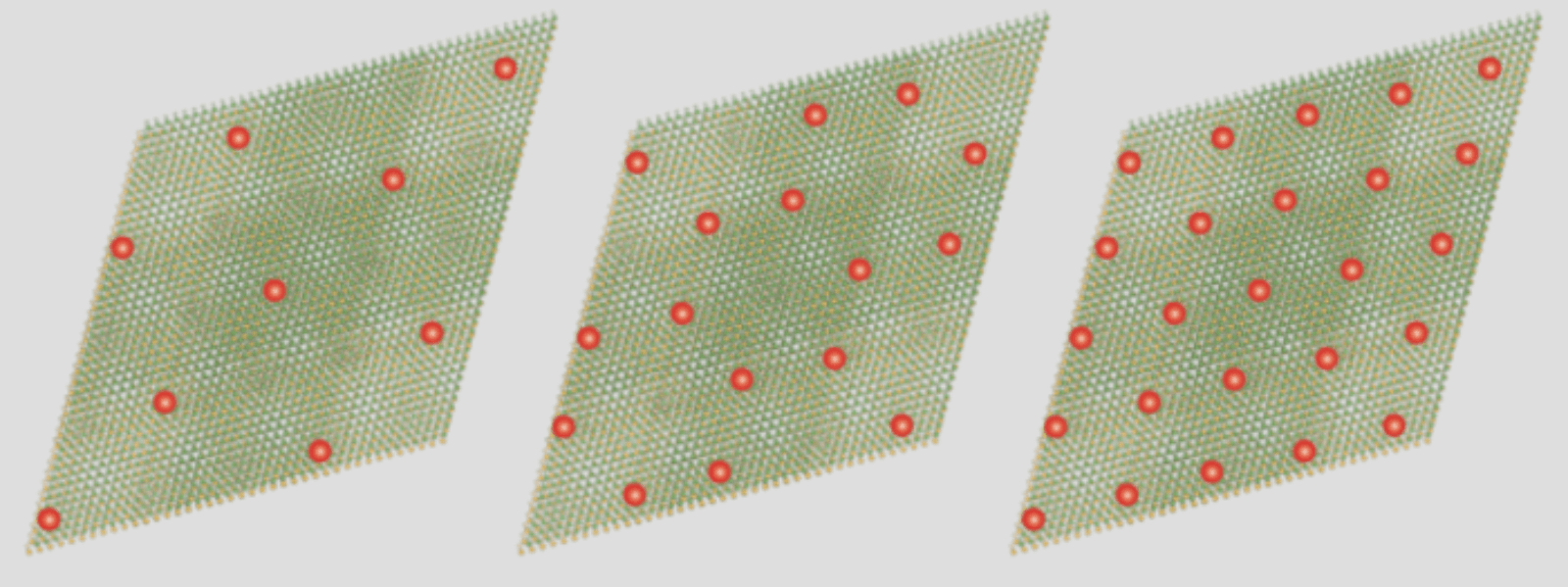
2D materials—which are no more than a few atoms thick—could lead to breakthrough electronics and quantum devices. Yet they are still not well understood. A recent study led by Feng Wang, a member of the Kavli Energy NanoScience Institute at Berkeley, has shed some light on how electrons interact with one another in a moiré superlattice, a 2D lattice made of one material topped with a 2D lattice of another material that is twisted so it is at an angle to the first. Ordinarily, electrons zip right through a material, but in this lattice, electrons will suddenly stop moving. Why? Because once electrons fill all the positions they can fill, they push away other electrons. Surprisingly, in this system, they only need to fill some of those positions. Like social distancers, they push electrons away from nearby positions, too. Such exotic behavior could lead to new types of electronic devices as well as a better understanding of how 2D materials behave.
DNA toys, spoons, and clothing?
You have probably seen plastics made from renewable resources like organic sugars, starches, and cellulose. Now add DNA to the list—and then give it a gold star because DNA is more than just another chemical building block. It is a complex molecule that scientists can tweak to give it new properties, such as luminescence, conductivity, or the ability to stick to non-stick Teflon at temperatures down to -20* C. DNA is everywhere, and it is much easier to turn into plastic than other biomolecules. Instead of a series of complex chemical reactions, researchers remove it from plants, bacteria, algae, or even salmon with ethanol, dissolve it in water, and add a single chemical to turn it into a polymer. Remove the water and it forms a plastic or a glue. According to Dan Luo, a member of the Kavli Institute at Cornell, the process could be economical at industrial scale.
Kavli members win major recognition
The American Chemical Society (ACS) has awarded the 2021 Priestley Medal, its highest honor, to Paul Alivisatos, founding director of the Kavli Energy NanoScience Institute at Berkeley. It recognizes his “foundational contributions” in nano chemistry. These include the development of nanocrystals and quantum dots now used in high-resolution displays, medical imaging, and renewable energy. Alivisatos is also cited for his leadership in the chemistry and nanoscience communities. He is currently executive vice chancellor and provost of University of California, Berkeley, and a former director of Lawrence Berkeley National Laboratory.
Meanwhile, Nynke Dekker has been awarded the NWO Spinoza Prize, one of the two most prestigious awards in Dutch science. Dekker is a professor of biological physics at the Kavli Institute of Nanoscience at Delft. She is best known for applying techniques used to study physics to understanding the function of individual DNA and RNA molecules and the proteins they express in bacteria, viruses, and eukaryotes. She has described, for example, how virus proteins insert errors in viral RNA so they can mutate and survive changing environments, and how antiviral inhibitors disrupt these proteins.